Introduction: Race significantly influences phenotypic and genetic diversity in hematological diseases, consequently affecting disease prognosis, Human Leukocyte Antigen-matching and Hematopoietic Stem Cell Transplant (HSCT) outcomes. To ensure the development of novel transplant strategies effective for patients from diverse backgrounds, all racial groups must have equal opportunities to take part in HSCT clinical trials.
Methods: Using ClinicalTrials.gov, a web-based resource that registers all studies meeting the definition of a clinical trial according to the International Committee on Medical Journal Editors, we identified all phase 1 to phase 4 HSCT clinical trials registered from 2006 to 2022. We manually abstracted data on the racial distribution of enrolled participants, sex distribution, transplant type, trial phase, location, and year of trial reporting. We calculated observed ratios of racial participation and compared them with the estimated probability of finding adequate donors from different racial subgroups reported by the National Marrow Donor Program to define the disproportionate enrolment factor. We then conducted subgroup racial distribution based on the trial phase, location, and type of transplant. We further evaluated trends in racial disparity within clinical trial enrolment over the years.
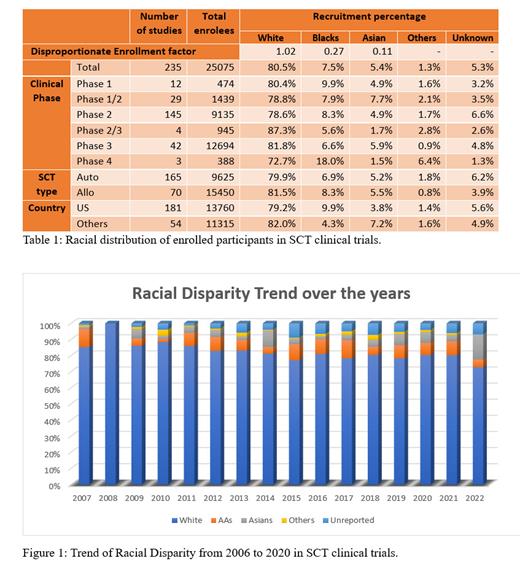
Results: We identified 398 clinical trials related to HSCT between 2006 and 2022, of which 72% were allogenic and 28% were autologous HSCT trials. Most of these were phase 2 clinical trials (64.3%) and the majority (78.1%) were conducted in the United States. The racial distribution of enrolled participants was publicly available in 235 (59%) of these clinical trials, including 57.7% allogeneic and 62.5% autologous HSCT. The race distribution of enrolled subjects was reported in 80% of Phase 1 and 79% of Phase 3, whereas only 48% of Phase 1/2 and 44% of Phase 2/3 clinical trials reported racial distribution. The race distribution among 25,075 study participants in 235 clinical trials was: 80.4% Whites (20,175), 7.4% Blacks (1858), 5.3% Asians (1336), 1.6% other races (396), and 5.3% (1310) subjects with unreported race. The same race distribution trend was observed across all subgroups of clinical trials based on phase, location, and transplant type (Table 1). The disproportionate enrolment factor was 0.27 for Black participants, and 0.11 for Asian participants, compared to 1.02 for Whites. From 2006 to 2022, yearly trends for the absolute number of clinical trial enrollees showed a 2.5% and 1.5% increase in Black and Asian subject representation; however, minorities still accounted for only 20.3% of total representation in 2022 (Figure 1).
Conclusions: Our results emphasize the significant racial underrepresentation of minority populations with a very low disproportionate enrolment factor in HSCT clinical trials despite the increased probability of finding a suitable donor resulting from novel HSCT technologies in recent years. Addressing these disparities requires urgent action and a multi-faceted approach. Efforts such as targeted outreach, cultural competence training and community engagement to focus on eliminating barriers are urgently needed to increase the enrolment of the minority population to achieve racial equity in HSCT.
Disclosures
Brunstein:Consulting for Allovir: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal